Innovative Scaffold: Beta-TCP, Autologous Fibrin, Stem Cells

Innovative Scaffold Solution for Bone Regeneration Made of Beta-Tricalcium Phosphate Granules, Autologous Fibrin Fold, and Peripheral Blood Stem Cells
Ciro Gargiulo Isacco, Kieu C. D. Nguyen, Andrea Ballini, Gregorio Paduanelli, Van H. Pham, Sergey K. Aityan, Melvin Schiffman, Toai C. Tran, Thao D. Huynh, Luis Filgueira, Vo Van Nhan, Gianna Dipalma, and Francesco Inchingolo
1. Introduction
Diseases, trauma, and surgical procedures can be the cause of bone paucity and defects. Due to the complexity of bone anatomy and physiology bone tissue degeneration and diseases can pose a big threat to doctors and physicians. However, modern bone tissue biomedical engineering has been con¬sidered as a valid substitute solution for these con¬ditions [1]. Procedures applied to repair defects or degeneration need the use of proper biomaterials with the right dimensions and anatomy shape that can fit into the damaged area. The cases of larger and more difficult defects need highly osteogenic scaffolds to promote and improve the bone tissue formation and regeneration [2].
The majority of scaffolds made of ceramics and metals or from polymers showed a weak osteo¬genic capability. Bone grafts are currently avail¬able in many different forms, such as allogeneic/ autologous demineralized bone matrix or implants, growth factor-loaded microbeads, or bio-derivate as calcium hydroxyapatite, gels, ceramic derivate, sea corals, and metals [2, 3]. The main target is to reduce the incidence of collateral complications such as rejection, infection and inflammation, and donor-site morbidity and of course reduce the overall costs and related expenses such as frequent hospitalization [3-5]. Breakdowns, such as partial ruptures or complete collapse, are the major issues related to synthetic implants, generally due to quality of the material and subtle autoimmune responses that may also create ideal conditions for bacterial growth, inflammation, and rejection [6¬9]. Metal implants, for example, may cause mal¬function due to aseptic loosening with specific inflammatory and immune responses to metal¬wear particles released during bio-corrosion which intensify the osteolytic activity of osteoclasts at the bone-implant interface, leading to a progressive loss of fixation [8, 9]. Therefore, an optimal bio-material should possess specific bio-characteris¬tics and qualities that should be biodegradable, tolerable, and safely absorbed by the body. This should happen without causing any kind of dam¬aging event such as an inflammation or an immune reaction, capable of carrying and supporting tissue growth and proliferation, thus allowing bone regeneration [5, 9].
The latest generations of bio-implants have been created with the precise intent of functioning as cell carriers capable of reproducing human bone formation process. The newest generation of these types of scaffolds has been developed with materials that possess specific mechanical and structural properties that are compatible with the anatomical site into which they are to be inserted, with enough volume fraction and high surface area to carry an enough number of cells within the scaf¬fold and the surrounding host tissues. This allows ingrowth and vascularization [5]. Therefore, the new bio-implants tend to replicate the process of the formation of new bone development or which physiologically takes place after an injury [10].
An inflammatory response takes place after an implantation of a biomaterial as a consequence of host immune response [10]. During this phase monocytes differentiate to tissue macrophages. However, presence of MSCs promotes an immune- modulatory activity on macrophage M1/M2 bal¬ance towards M2 commencing a favorable cascade of events where interleukins such as IL-10, IL-4, IL-13, and IL-6 and prostaglandin E2 initiate the first step of the repairing process [11-14]. Bone plays a key role in well functioning of immune sys¬tem and it is the site that immune cells are created. In fact, autoimmune disorders often induce bone tissue damages and degeneration, an event that has been confirmed by an experiment where macro¬phage ablation leads to intramembranous bone defection and inhibiting of the healing process [14].
In effect, previous studies have shown that some biomaterials due to high similarity with human tissues are able to trigger physic-chemical signals leading to stem cell differentiation towards diverse cell phenotypes as osteoblasts [15, 16]. Results have shown that biomaterials based on calcium phosphate (CaP), a major constituent of native bone tissue, induce naive stem cells towards osteogenic differentiation promoting in vivo bone tissue formation and augmentation [16, 17].
However, though CaP is quickly absorbed in vivo, the process often occurs preceding the for¬mation of new bone tissue that results in an incon¬gruence between the host’s new bone and scaffold. Conversely, P-TCP seems to be better compatible as the absorption rate is slower with a steady release of both calcium (Ca2+) and sulfate (SO42-) ions [18].
In line with our published study, we can con¬firm that hPB contains the right amount of differ¬ent subsets of pluripotent and multipotent stem cells such as MSCs, HSCs, NSCs, and ESCs capable of differentiating into cells of different lineages such as osteoblasts [19]. In this current study, we have noted that part of hPB-SCs were induced to differentiate to active osteoblasts under the direct influence of P-TCP granules within a period of 7-10 days without the need of osteo-inductor medium in cell culture flask. Therefore we have hypothesized that the adjunct of autologous hPB-SCs together with p-TCP embedded in fibrin matrix gel could enhance both the correct time fraction for endogenous bone formation and the quality of the bone tissue itself in both in vitro and in vivo condition.
2. Material and Methods
2.1. Beta-Tricalcium Phosphate
The biomaterial composed of p-TCP (GUIDOR Calc-i-Oss) granules with diameters of 3 X 0.5ml 500-1000 pM were supplied by Sunstar Degrad¬able Solution AG Co., AS. Switzerland. The gran¬ules were irregular in shape, and each had interconnection diameters ranging from 70 to 200 pm. The porous structure of p-TCP was char¬acterized using a scanning electron microscope (SEM, Zeiss-Sigma, USA). The porosity and interconnections of the porous as well as the com¬position and weight percentage of p -TCP gran-ules were calculated using an analysis software package performed at Sunstar Degradable Solu¬tion AG laboratories. Guidor Calc-i-Oss consists of pure p-TCP with a purity of > 99% with a Ca/P molecule ratio of 1.5. Resorption will take place mostly parallel to bone regeneration. Depending on the regeneration potential of the host tissue, it will be completely resorbed within 9-15 months.
Peripheral blood 24 mL from each consent donor (n = 10) has been collected in four HSC VacutainerR tube kit (1 red, 1 green, and 2 whites (Human-Stem CellsR provided by Silfradent, Italy)). The red cap tube consists of internal coating material made by silicon crystals in a coagulum and when separation/centrifugation is done at room temperature the final product is in a condensed form. White cap tube has no material and after separation the fibrin gel will be ready after 20 min. Green cap tube contains heparin and Ficoll-Paque PLUS (GE Healthcare Life Science-Uppsala, Sweden) to which blood is added in a ratio of 1:2.
The procedure to obtain Compact Bio-BoneR scaffold includes the use of p-TCP granules mixed together with different preparations obtained from the blood collected in four VacutainerR tube kit. The blood needs to be centrifuged for 12 min at different variation speeds (Medifuge, Silfradent, Italy). In the first step, the clotted plasma-rich fibrin from the red cap tube is collected, separated from blood part, and squeezed to obtain fibrin serum, while the membrane was chopped into pieces and mixed with p-TCP granules and fibrin serum. Then, 2 mL plasma from the white cap tube without anticoagulant was used and mixed with p-TCP and fibrin, moved into Eppendorf conical tube 2 mL, and inserted in the APAG heater machine (Silfradent, Italy) for max 1 min at 74 °C. Meanwhile, peripheral blood stem cells (about 2 X 10 6 ± 5 X 105 cells/mL) from the green cap tubes were isolated by using one part of Ficoll-Paque PLUS and two parts of blood, gently washed twice with PBS, and injected directly into the p-TCP and fibrin composite precedently obtained. The scaffold was put in flasks contain¬ing 5 mL serum-free medium (SFM, Gibco, Germany) and incubated at 37 °C with 5% CO2; the medium was changed every 5 days.
2.2. Cytochemical Staining, Flow Cytometry Analysis, and RT-PCR
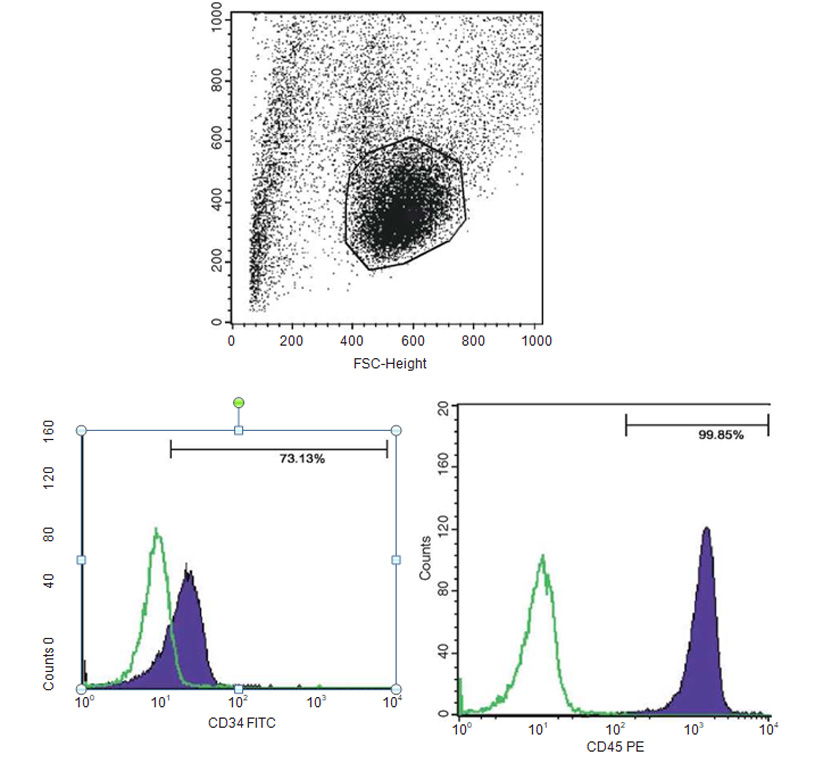
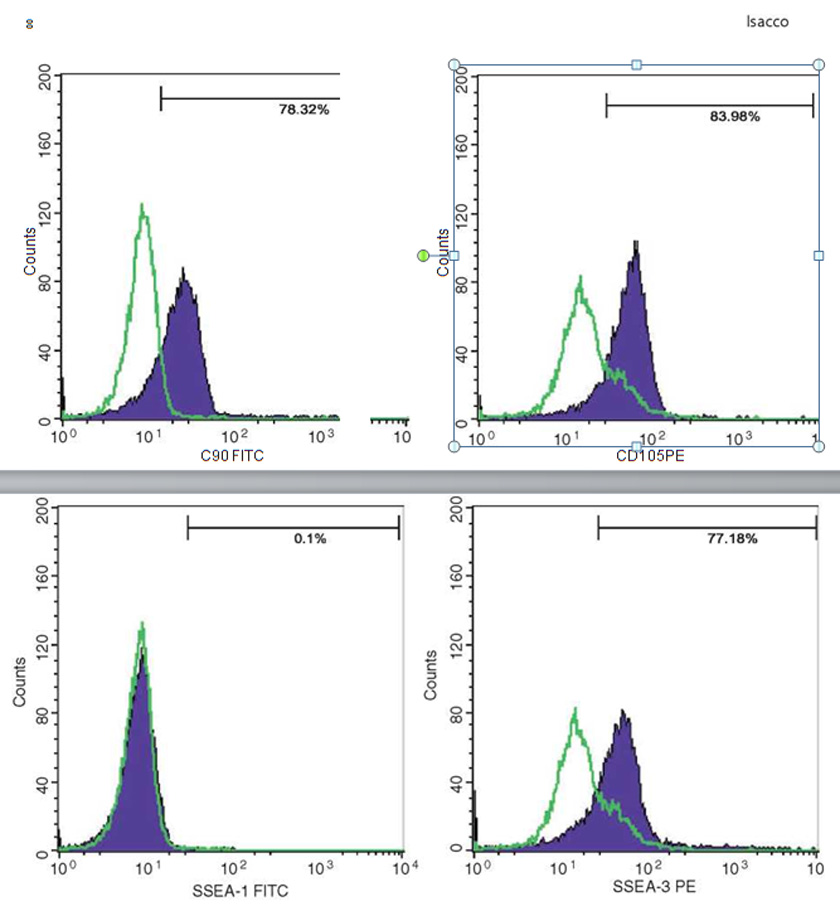
Mineral matrix deposits and bone nodules of osteoblasts, from human PB-SCs and es, were evaluated by staining cell cultures with alizarin red (AR), alkaline phosphatase (AP), and von Kossa (VK); flow cytometry analysis was used to confirm the expression of multipotent and plu¬ripotent stem cells such as CD34, CD45, CD90, CD105, and SSEA3.
2.3. Alizarin Red Stain Procedure
The presence of calcium deposits was detected by washing cells with cold PBS and fixing them in NFB-neutral formalin buffer solution 10% for 30 min in a chemical hood. Cells were rinsed three times with distilled water and immersed in 2% solution of alizarin red for 5 min. Cells were rinsed 2-3 times in distilled water and checked under inverse microscope and photographed.
2.4. Alkaline Phosphatase Stain Procedure
The presence of alkaline phosphates was detected by washing cells with cold PBS and fixing them in NFB-neutral formalin buffer solution 10% for 30 min in a chemical hood. Cells were then stained with solution naphthol As-MX-PO4 (Sigma) and Fast red violet LB salt (Sigma) for 45 min in the dark at room temperature. Cells were rinsed three times in distilled water and checked by inverse microscope and photographed.
2.5. Von Kossa Stain Procedure
The presence of calcium deposits was detected by washing cells with cold PBS and fixing them in NFB-neutral formalin buffer solution 10% for 30 min in a chemical hood. They were then stained with 2.5% silver nitrate (Merck, Germany) for 30 min in the dark. Cells were rinsed three times with distilled water, checked by inverse microscope, and photographed.
2.6. Cytochemical Staining of CFU-Fs
After being cultured in serum-free medium (SFM) for 7 days, PB-SCs were fixed with cold 10% neutral-buffered formalin (30 min at 4 °C) and then assayed for colony-forming unit fibro¬blasts (CFU-Fs). Briefly, the substrate solution was prepared by removing flasks from the incu¬bator. We removed off cell culture medium and washed the flask with 10-15 mL of PBS. We added 10 mL of a 0.5% crystal violet solution (Sigma) made with methanol and we incubated the dishes, on the bench at room temperature, for 30 min. Then, the crystal violet solution was removed and the flasks were carefully rinsed 4x with 10-15 mL D-PBS. Then, each flask was gently rinsed 1x with tap water. All remained tap water was accurately pipetted off and let dry. The colonies were enumerated by microscope.
2.7. Immunophenotyping by Flow Cytometry Analysis
Immunophenotyping by flow cytometry analysis was performed to evaluate the presence of a set of markers CD34, CD45, CD90, CD105, and SSEA3. Cell samples were washed twice in Dulbecco’s PBS containing 1% BSA (Sigma-Aldrich). Cells were stained for 30 min at 4 °C with anti-CD14- fluorescein isothiocyanate, anti-CD34-fluorescein isothiocyanate, anti-CD133-fluorescein isothiocy-anate, anti-CD44-phycoerythrin, anti-CD45-fluo- rescein isothiocyanate, anti-CD90-phycoerythrin, anti-CD105-fluorescein isothiocyanate mAb, anti-Nestin, anti-Tra1, and anti-SSEA3 (BD Biosciences, Franklin Lakes, NJ, USA). Stained cells were analyzed by a FACSCalibur flow cytom¬eter (BD Biosciences). Isotype controls were used for all analyses.
2.8. Real-Time-Polymerase Chain Reaction Procedure
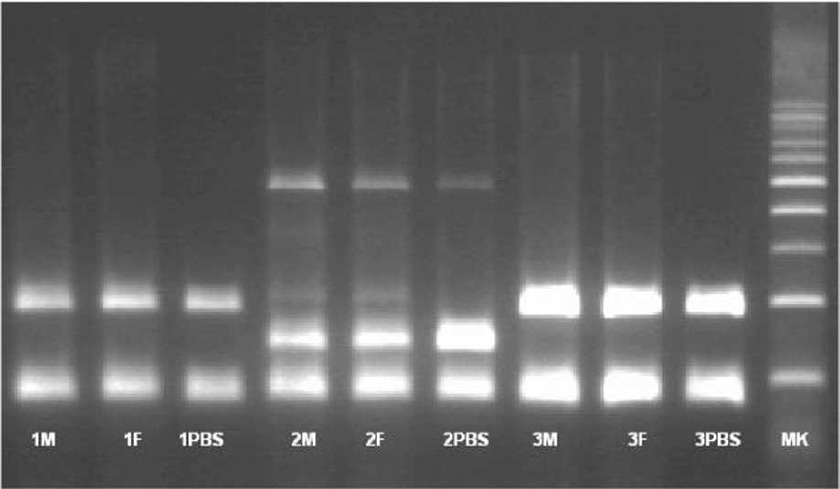
RT-PCR for the expression of OCT-4, Sox-2, osteocalcin, Nanog, Nestin, DMP, and GAPDH was performed on human PB-SC-derived osteo¬blasts. Results were confirmed using a negative control procedure. Total RNA extraction of the tested cell culture was carried out including the positive control using the Trizol LS reagent (Invitrogen): (1) 150 pL of cell culture was added to one biopure Eppendorf tube containing 450 pL Trizol LS (Invitrogen) and homogenized by mix¬ing up and down several times. (2) The homoge¬nized solution was incubated at 30 °C for 10 min. (3) 120 pL of chloroform was added, then rapidly and carefully shaken for 15 s, kept at 30 °C for 10 min, and centrifuged in a refrigerated centri-fuge (2-8 °C) at 1300 x g for 15 min. (4) 300 pL of the supernatant was carefully transferred into another biopure Eppendorf tube. While taking care not to disturb the interface, 300 pL isopropa¬nol was added, and the tube was shaken gently up and down for a few seconds, then kept at 30 °C for 10 min, and centrifuged in the refrigerated centrifuge at 1300 X g for 20 min. (5). The super-natant was removed without losing the precipi¬tated RNA (sometimes invisible) at the bottom or on one side of the bottom of the tube. (6) About 1 mL ethanol 80% was added without any shak¬ing, and then centrifuged in the refrigerated cen¬trifuge at 800 X g for 5 min; all of the supernatant was removed using a vacuum pump. (7) The pel¬let was dried at 55 °C for 10-15 min; 40 pL Q water is added to the dried pellet and then kept at 56 °C for 10 min. The isolated RNA was stored at -20 °C prior to RT-PCR. The cDNA synthesis was carried out using the iScript cDNA synthesis kit (BioRad): (1) In one tube PCR 0.2, 1 pL reverse transcriptase was added, 4 pL RT mix, and 15 pL of the isolated RNA; this was gently mixed by pipetting up and down several times. (2) The cDNA synthesis was carried out in the thermal cycler by the following thermal cycle, 25 °C/5 min, 42 °C/30 min, and 85 °C/5 min, and then kept at 4 °C until PCR (Table 13.1).
2.9. Primers
DMP
GCAGAGTGATGACCCAGAG sense primer (3' to 5')
GCTCGCTTCTGTCATCTTCC antisense primer (5' to 3')
200 Primer expected size (bps)
OCN
CAAAGGTGCAGCCTTTGTGTC sense primer (3' to 5')
TCACAGTCCGGATTGAGCTCA antisense primer (5' to 3')
150 Primer expected size (bps)
Nestin
AGAGGGGAATTCCTGGAG sense primer (3' to 5')
CTGAGGACCAGGACTCTCTA antisense primer (5' to 3')
496 Primer expected size (bps)
RUNX2
GGTTAATCTCCGCAGGTCACT sense primer (3’to 5')
CACTGTGCTGAAGAGGCTGTT antisense primer (5' to 3')
203 Primer expected size (bps)
GAPDH
CCCATCACCATCTTCCAGGA sense primer (5' to 3')
TTGTCATACCAGGAAATGAGC antisense primer (5' to 3')
94 Primer expected size (bps)
3. Discussion
The improved understanding of the tissue micro-environment where the replacements had to be done resulted in better quality of the biomaterials used for the generation of bone implants. Early graft substitutes were conceptually made respect¬ing the mechanical properties of the affected area; therefore biomaterial should have matched the physical properties of the replaced structure while keeping integrity with the surrounding environ¬ment [20]. Metals, ceramics, and polymers were the main materials used in these procedures. The collateral was the presence of consistent idiopathic immune responses probably due to the formation of fibrous tissues at the biomaterial-tissue interface that led to aseptic loosening. The persistent inflam¬matory response induced the generation of fibrotic connective tissue that encapsulated the foreign structure repairing it from the attack of immune system. This generated a deep friction between the implant and the surrounding tissues that eventually led to the total removal of the implant [20].
Thus second generation of bone graft substitutes were studied with the intent of inducing specific biological responses improving osteoconduction and vascularization to avoid the formation of this fibrous layer by using bioactive and biodegradable coatings. The procedure based on the use of bio¬components that promote the integration with the surrounding tissues such as bioactive ceramics to include HA, P-TCP, or bioactive glass that closely mimics the bone tissue microenvironment [20]. These bio-compounds were designed to allocate stem cells and growth factors enhancing bone repair and regeneration and providing the necessary cel¬lular and molecular support for vascularization and enough nutritional support [20].
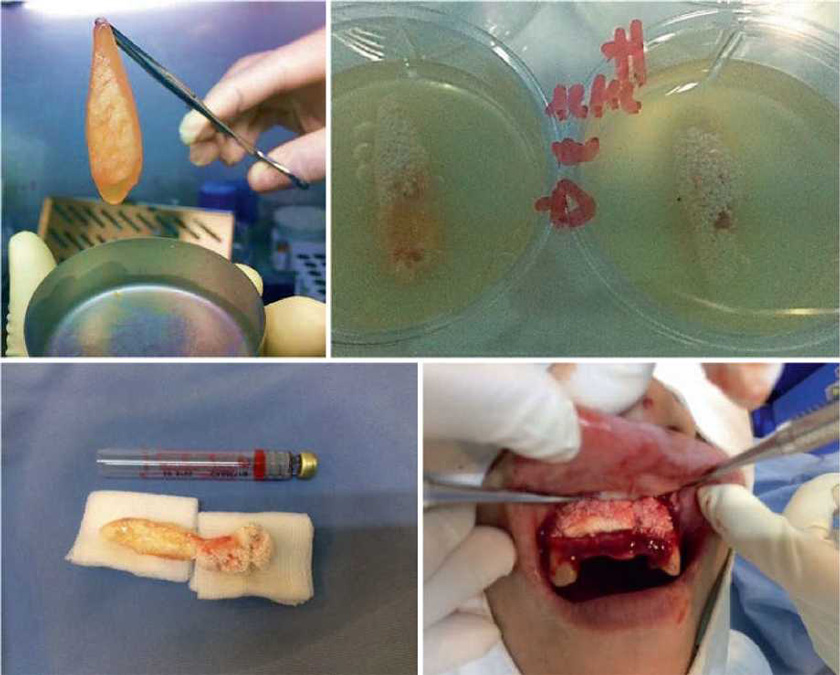
We have presented through this chapter a new bioscaffold that meets the basic needs essential to enhance the repair, regeneration, and growth of host bone tissue, easy to manipulate and insert in in vivo procedures as presented in (Figs. 13.14, 13.15, and 13.16). This new scaffold due to the combined presence of autologous fibrin gel matrix, P-TCP, and autologous PB-SC-derived osteoblasts closely mimics the bone tissue micro¬environment. The in vitro phase showed few prominent points that allow us to predict that this new bioscaffold may eventually match the bio¬material degradation rate with the bone regenera-tion rate and also a proper control of stem cells growth over their release kinetics and it may induce enough vascularization on the site.
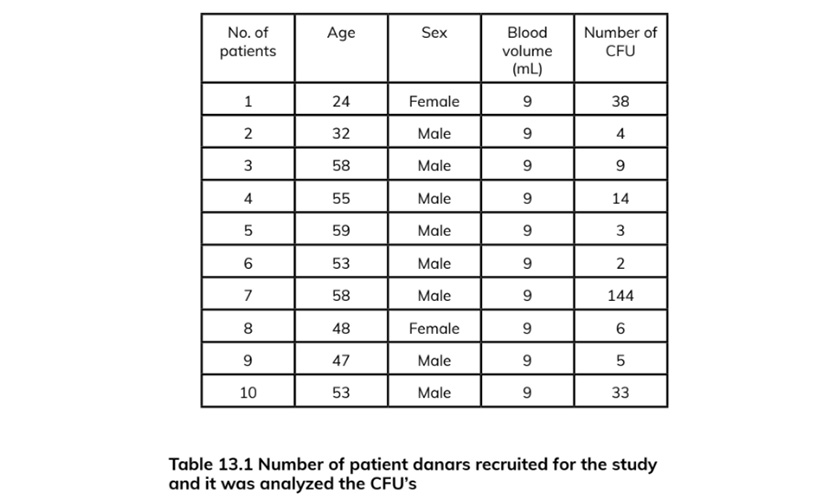
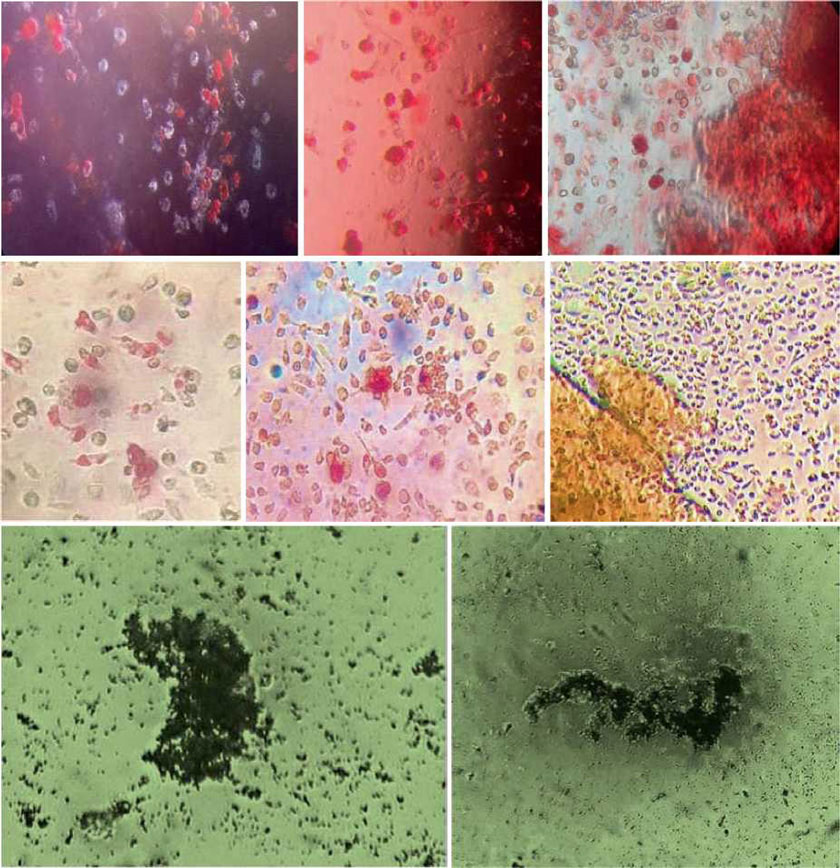
The presence of pluripotent and multipotent PB-SCs both adherent and floating was tested by flow cytometry analysis and resulted posi¬tive for markers like CD 34-45-90-105 and SSEA-3 but negative for SSEA-1 (Figs. 13.18, 13.19, 13.20, 13.21, 13.22, and 13.23). The autologous PB-SCs obtained by consent donors were seeded with P-TCP embedded in a gel of fibrin gel obtained by donor’s blood and were named the Compact Bio-Bone. Compact Bio¬Bone. Compact Bio-Bone was positively tested by specific immune stain like alizarin red (AR, Figs. 13.1, 13.2, and 13.3), alkaline phospha¬tase (AP, Figs. 13.4, 13.5, and 13.6), and von Kossa (VK, Figs. 13.7 and 13.8); the Compact Bio-Bone cells were then tested by RT-PCR for the expression of osteo-matrix producing genes and the outcomes were positive for Runx2 (203 Kb), OCN (150 kb), DMP (200 Kb) and Nestin (496 Kb) (Fig. 13.13); eventually the scaffold was pictured in high resolution by SEM (Figs. 13.9, 13.10, 13.11 and 13.12). These results though obtained from in vitro showed highly promising outcomes especially if one considers the use in bone reconstructive proce-dures; the presence of mature osteoblast-like cells in a relatively short period of 7-12 days not only prefigures the possibility of a faster resorption rate once applied in vivo, but also definitively opens up the chance to resolve the formation of better endogenous bone forma¬tion. The presence of Nestin detected within Compact Bio-Bone cells, as recently confirmed by other authors though from bone marrow- derived stromal cells, showed to contribute to the normal osteoblast lineage cell turnover in the adult bone [21] (Figs. 13.19, 13.20, 13.21, 13.22, and 13.23).

4. Conclusion
The big majority of researches in the develop¬ment of these types of biocompatible bone graft substitutes are still in the experimental testing phase and very few have been conducted on clini¬cal scale. A large clinical phase testing will be a necessity as it will open a new vision about the complexity of physiological-molecular processes that are involved in the clinical scenario. In addi¬tion, there is still much to learn about the strict relationship between bone tissue microenviron¬ment and the current endocrine-metabolic condi¬tion of the patient to assess a proper individualized treatment and procedure. All of these questions surely will need a close interdisciplinary approach between all the different branches of science as medicine, chemistry, physics, and engineering.
References
1. Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol. 2005;94:1-22.
2. Ling JL, Liu N, Shi JG, Liu Q, et al. Osteogenic scaf-folds for bone reconstruction. BioResearch Open Access. 2012;1(3):137-44.
3. Tran CT, Gargiulo C, Thao HD, Tuan HM, Filgueira L, Michael Strong D. Culture and differentiation of osteoblasts on coral scaffold from human bone mar¬row mesenchymal stem cells. Cell Tissue Bank. 2011;12(4):247-61.
4. Fisher JN, Peretti GM, Scotti C. Stem cells for bone regeneration: from cell-based therapies to decellular- ised engineered extracellular matrices. Stem Cell Int. 2016;2016:1-15.
5. O’Brien FJ, Farrell E, Waller MA, Connell I, et al. Scaffolds and cells: preliminary biomechanical analy¬sis and results for the use of a collagen gag scaffold for bone tissue engineering. Topic Bio-Mech Eng. 2004:167-83.
6. Einhorn TA. Enhancement of fracture healing. J Bone Joint Surg. 1995;77:940-56.
7. Puska M, Aho AJ, Vallittu P. Polymer composites for bone reconstruction. Adv Compos Mater Anal Nat Man-Made Mater. 2009;3:55-74.
8. Carlsson AS, Magnusson B, Moller H. Metal sensitiv¬ity in patients with metal-to-plastic total hip arthro¬plasties. Acta Orthop Scand. 1980;51(1):57-62.
9. Thomas P, Thomsen M. Allergy diagnostics in implant intolerance. Orthopedics. 2008;37(2):131-5.
10. Gamblin AL, Brennan AM, Renaud A, Yagita H, Lezot F, et al. Bone tissue formation with human mes¬enchymal stem cells and biphasic calcium phosphate ceramics: the local implication of osteoclasts and macrophages. Biomaterials. 2014;35:9660-7.
11. Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11e20.
12. Adutler-Lieber S, Ben-Mordechai T, Naftali-Shani N, Asher E, Loberman D, et al. Human macrophage regulation via interaction with cardiac adipose tissue- derived mesenchymal stromal cells. J Cardiovasc Pharmacol Ther. 2013;18:78e86.
13. Nemeth K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42e9.
14. Alexander KA, Chang MK, Maylin ER, Kohler T, et al. Osteal macrophages promote in vivo intramem- branous bone healing in a mouse tibial injury model. J Bone Min Res. 2011;26:1517-32.
15. Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mes¬enchymal stem cells. Nat Mater. 2008;7(10):816-23.
16. Shih YRV, Hwang YS, Phadke A, Kang H, et al. Calcium phosphate-bearing matrices induce osteo¬genic differentiation of stem cells through adenosine signaling. PNAS. 2014;111(3):990-5.
17. Peng G, Haoqiang Z,Yun L, Bo F, et al. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci Rep. 2016;6:23367.
18. Chang YL, Stanford CM, Keller JC. Calcium and phosphate supplementation promotes bone cell min-eralization: implications for hydroxyapatite (HA)- enhanced bone formation. J Biomed Mater Res. 2000;52(2):270-8.
19. Gargiulo C, Pham VH, Hai NT, Nguyen NCD, Pham VP, Abe K, Flores V, Shifman M. Isolation and char-acterization of multipotent and pluripotent stem cells from human peripheral blood. Stem Cell Discov. 2015;5(3):1-17.
20. Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15-56.
21. Panaroni C, TzengYS, Saeed H, Wu JY. Mesenchymal progenitors and the osteoblast lineage in bone mar¬row hematopoietic niches. Curr Osteoporos Rep. 2014;12(1):22-32.

Bài viết liên quan
- Mất 1 răng hàm có sao không?
- Tác hại của mất răng hàm và giải pháp khắc phục
- Bị mất răng hàm - Nguyên nhân và cách điều trị
- Bảo hành trồng răng Implant tại trung tâm Implant Việt Nam
- Phục hình cầu răng trên trụ implant là gì?
- Trồng răng Implant không đau tại Implant Việt Nam
- Ưu điểm trồng răng trả góp tại Trung tâm Implant Việt Nam
- Tất tần tật những điều cần biết về implant
